
Road salt can make our winter roads safer to drive. Photo by Pexels (Pixabay: Link)
It’s that time of year when you see metre high piles of road salt at the local shop for five bucks a bag. This means it is also time for crunchy sidewalks, dog booties, and salt-stained boots. Not only that, but road salts have a less obvious but severe impact on local waterbodies.
Now, let’s take a trip down memory lane, all the way back to grade school chemistry. When road salt mixes with water it dissolves into sodium chloride (Na-Cl). So, when it rains or there is a lot of snowmelt, the salt turns to sodium chloride which flows directly into our rivers, lakes, and streams. This extra salt causes spikes in salinity in the freshwater.
Impact on Aquatic Life
In Ontario, we use road salt for de-icing sidewalks, roads, and parking lots and to improve safety during winter temperatures. The problem is that it is often used in excess, turning freshwater into saltwater.
The salty waters can flow into groundwater supplies, killing sensitive species, and ultimately degrading ecosystems. Some spots in Ontario have become so salty that there have been sightings of saltwater animals in the freshwater creeks. Reports of saltwater blue crabs living in Mimico Creek is just one troubling case that illustrates the extent of sodium chloride pollution in the Great Lakes watersheds.

A saltwater blue crab on the shores of Lake Ontario. Photo by Emma Lagunday (Flickr: Link)
With all of this added salt, freshwater species are increasingly at risk from spikes in sodium chloride levels in winter months. Research shows the salinization of freshwater causes increased parasitism on the northern leopard frog, a native amphibian to Ontario. In salmon species, sodium chloride stops growth at the most important life stage, which corresponds with drops in salmon population sizes.

A northern leopard frog floats in shallow swamp water in Clarence, NY. Photo by ~Sage~ (Flickr: Link)
In the worst cases, salinization of freshwater leads to mortality in small aquatic species like freshwater shrimp, mussels, and insects. Freshwater ecosystems rely on these animals because they feed larger animals like fish and birds.
When populations of small aquatic animals drop as sodium chloride levels increase, the population of larger aquatic animals also drop. This causes major changes to the food web and damages the overall aquatic ecosystem. All-around road salts cause permanent changes to our aquatic ecosystems, including a tremendous loss in biodiversity.
How much road salt is enough?
In Ontario, the solution right now is to use less road salt on driveways, sidewalks, and paved parking lots. The over-application of road salt is common and unnecessary. The illustrations below show the average rate at which the Ministry of Transport applies to roads (top left) and the over-application typically seen in parking lots (bottom right).
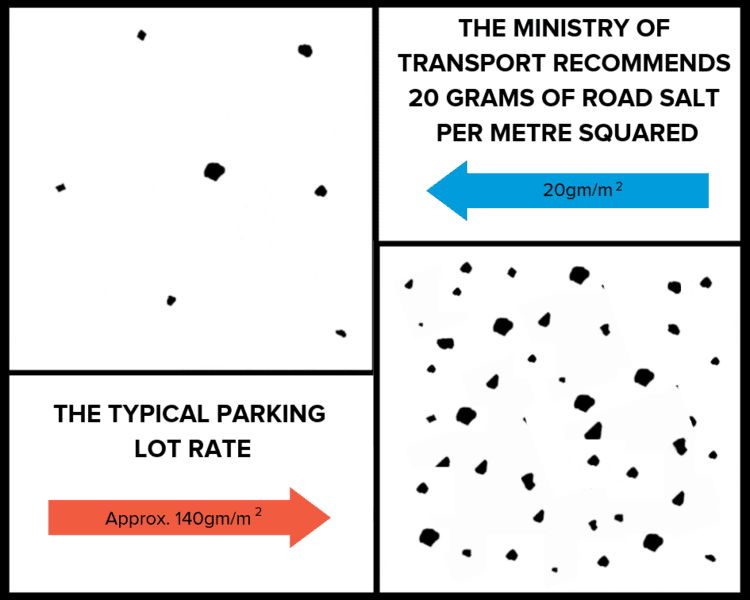
This illustration is from a presentation by Tim Van Seters on the Lake Ontario Evening, November 22, 2018, titled Salting the Earth: The Unintended Impacts and What We Can do to Prevent Them.
Most of the public is simply unaware of the impacts that road salts have on the local environment. Plus road salts are really cheap and there is a general lack of application protocol and enforcement. These factors often leave us prone to excessive application during the freezing winter months.

A mixture of 20% sugar beet juice, a byproduct of beet sugar production, and 80% salt brine enables the salt to work at lower temperatures. Photo by Viviane6276 (Pixabay: Link)
Every winter the topic of road salt appears on a variety of blogs and media outlets. Lots of research continues to look for alternatives to road salts, including beet brine which is currently being used in Calgary.
Actions that you can take:
If you can avoid using salt altogether, great! If you must use road salt, here are some easy tips to reduce your use and protect our lakes:

One lucky Michigan man uses a snowblower to get the job done. Photo by JillWellington (Pixabay: Link)



